
Note: Phage research is a rapidly evolving field. The data and findings I cite in this piece are current as of Feb 8, 2025. To better understand these concepts, I always recommend reading the latest literature directly.
Table of Contents
The Elephant Under the Rug
Phages in Transit
Phages And the Immune Lullaby
Phages Strangely Avert Antibodies
Distilling our Evidence
Synthesizing Theories
Phaging the Future
The Elephant Under the Rug
Somehow, despite all the controversies and camps that cut across the phage world, almost everyone seems to agree that phages are safe.
And the evidence seems to support it. After more than half a century of human use, our largest trials and meta-analyses show that phage therapy is consistently well-tolerated, and certainly more so than traditional antibiotics (Pirnay et al., Kim et al., Brüssow et al.)
Everyone seems to know that phages are safe. Hardly anyone seems to know why.
Phages might be longtime residents of the human microbiome, but they’re still viruses that are distinct from our tissues. Which begs the question: how come phages don’t trigger nearly the same immune response as other viruses or pathogens?
To me, rabbit holes like these are fun because they’re bound to produce interesting answers. If phages did activate the human immune system, why doesn’t phage therapy produce any macroscopic effects? If, on the other hand, phages did evade our immune responses, how does that work?
Whatever the answer turns out to be, my hunch is that it’s going to be meaningful.
In this article, I want to synthesize our most reproducible results from over a dozen studies on phage-immune interactions. And using the insights that emerge, I want to ask sharper questions that inform future experiments.
Phages in Transit
Naturally, the question of how our immune system interacts with phages begins with the question of how phages are distributed across the body.
In some sense, this almost seems trivial as a starting point, but it’s incredibly relevant.
For one, phages subjected to real-time labeling experiments show distinct bio-availabilities based on their identity, morphology, concentration, administration route, and more (Dabrowska et al., Abedon et al.)
Adding to this complexity is the fact that phages display unpredictable tropisms over a variety of tissues.
As illustrated in a review by Kang et al., phages administered intravenously show asymmetric retention in a laundry list of organs—from the lungs, spleen, heart, and peritoneum1

Other authors show that the pathways of phage metabolism and excretion are just as varied, with orally administered phages being cleared through kidneys, spleen, or the mononuclear phagocytic system (Dabrowska et al., Zatzman et al. )
In short, the pharmacodynamics of phages is glaringly underrated.
If we buy into the idea that phages accumulate in a wide spectrum of tissues, it follows that they would also be exposed to vastly different environments that modulate their immune interactions.
Speed also seems to matter here. Interestingly, pharmacokinetic experiments report massive variability in both the speed and route through which phages are eliminated. Whereas phages like T4 show systemic clearing in minutes, phages like PA01 can exhibit half-lives that resemble those of longer-lived small-molecule drugs (Kang et al., Nang et al.)
From a basic intuition of input and output rates, I imagine that this is also important. For instance, if the residence period of a phage in the body is shorter than the time it takes to recruit macrophages or churn out cytokines, it isn’t unreasonable to assume that we don’t present with a visible immune response.
That said, kinetics alone is probably not the mechanism that defines our immune response to phages.
A clear exhibit of this lies in the interaction of phages with macrophages and phagocytes. Several studies show that phages like Pf4 are routinely engulfed by phagocytes, while a minority of phages co-opt routine processes like transcytosis and receptor-mediated endocytosis (Barr et al., Lin et al.).
Meanwhile, large-scale analyses show that phages routinely enter non-immune cells, including fibroblasts and epithelial cells (Champagne-Jorgenson et al.)

If phages are routinely taken up by our cells, they might linger long enough to trigger an immune response.
Of course, this argument isn’t airtight. Phages may be regularly engulfed and absorbed, but is this truly enough to trigger the following steps of an immune response?
At a glance, this seems true.
Notably, molecular studies show that while macrophages don’t upregulate the expression of antigen-displaying molecules, peptide fragments from a wide spectrum of lytic phages are found on MHC-II (and sometimes cross-presented on MHC-1) receptors of macrophages and dendritic cells (Górski et al.).
Had phages never reached bloodstream, or had they been cleared with blinding speed, we wouldn’t expect to find them sliced up and decorated on phagocytes.
But this should be even more surprising: if phages are pounced on and processed by innate immune cells, why don’t they spark the same innate immune responses that human viruses do?
Phages And the Immune Lullaby
Let’s take stock of what we’ve learned so far.
Our findings from earlier seem clear enough: the fact that phages can enter phagocytes at all suggests that they also bind to pattern recognition receptors (PRRs) on their surface.
Of course, this enables phage detection and engulfment, but it also sets off the classic chain of events that defines the innate immune response.
After detecting an antigen via PRR receptors, cells release a slew of cytokines, leading to vessel dilation, immune cell migration, and eventually the adaptive immune response.
The phenomenon we’re observing seems to be an interesting edge case. When the antigen in question is a phage, one could argue that this process is modulated, or perhaps even prevented from carrying out to completion.
Curiously, several human and murine trials converge on the finding that phage administration doesn’t just produce a neutral response, but actually lowers inflammatory cytokines like TNF-A and IL-6 (Dabrowska et al., Fongaro et al.). With few exceptions, this observation holds surprisingly well across a range of phage formulations and use cases2.
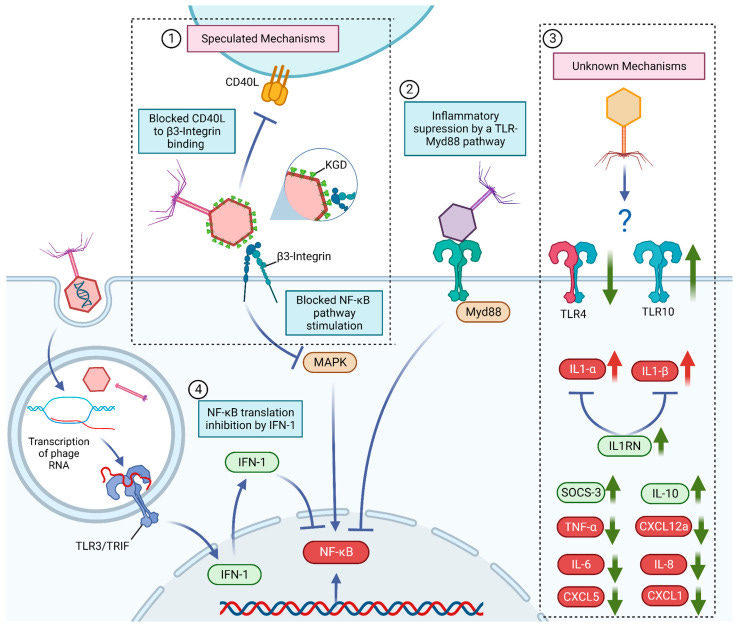
In parallel, work from Górski et al. demonstrates that several phylogenetically distinct phages can produce similar, predictable cytokine profiles in humans. This includes the same uncanny downtick in inflammatory markers like lysozyme and TGF-B, accompanied by a simultaneous surge in interferons (Kleinschmidt et al.)
The resulting image is blurry and somewhat contradictory. On one hand, the presence of interferons proves that our cells certainly aren’t blind to phages. On the other, phages seem to be dulling and delaying the inflammatory response that follows.
In vitro studies on phage-immune interactions offer some clarity. Notably, phagocytes co-cultured with phages are significantly less likely to engulf bacteria and other pathogens, even when the phages are no longer present.
Other studies, such as those from Mankiewicz et al., showed that prior exposure to phages in guinea pigs could significantly dampen their inflammation against a series of unrelated antigens, including tuberculin and other antigens.
This effect has been discussed for several years, and appears directionally reproducible with other phages and inflammatory agents (Przerwa et al., Souza et al.)

On some dimensions, this also aligns with the clinical history of phage therapy. Given the longstanding use of phages in human trials with minimal inflammatory response (i.e. fever, erythema, etc.), it isn’t unreasonable to assume that phages exert an inhibitory immune effect that renders them fundamentally different from other human viruses (Pirnay et al., Kim et al., Brüssow et al.).
Together, these observations independently point to the idea that phages may inhibit the innate immune response.
While the mechanisms of this inhibition remain unknown, it may explain why phages not only suppress immune responses against themselves and their hosts, but generate a field effect of sorts that also masks other, completely distinct antigens.
But perhaps there’s more at play here. If inflammation really is the trigger that fires off the immune cascade, then the anti-inflammatory effect that phages exert should compromise all the steps that follow — from the production of antibodies, to the activation of CD8+ Th cells, all the way down to the formation of immunological memory.
In any complex biological network, a single perturbation can perturb the entire system. Even if phages affected just a single point of immunity, we should expect them to influence the entire immune cascade.
But is this true? To what extent does our reasoning map with reality?
Phages Strangely Avert Antibodies
If we want to reliably measure how phages affect the adaptive immune response, perhaps the most obvious endpoint would be antibodies.
The current literature leads us to an unexpected, yet surprisingly reproducible finding. Namely, it seems that many of us already produce specific antibodies against an assortment of phages.
Through immunochemical assays, Jerne et. al and Majewska et al. reported that a non-trivial fraction of patients in their trials exhibited IgG and IgM antibodies to Escherichia phage T4 and T7 before ever receiving phages therapeutically. Further characterization revealed that many of the antibodies were specific to major capsid proteins, including Hoc and gp24.
Studies on a spectrum of other phages, including Staph cocktails like MS-1, have produced similar results. Despite a generally neutral response, or a small fraction of patients mounting antibody response, antibodies were not associated with detectable drop in efficacy of treatment (Zazcek et al.)
This shouldn’t be a stretch to imagine. Since Escherichia and Staphylococcus are already key residents of the human microbiome, there’s no guarantee if this trial really was these patients’ first exposure to T4 or MS-1.

If anything, the surprise doesn’t lie in the existence of antibodies, but rather their amount and action.
As the authors noted in their discussions, serum antibodies in these patients were detectable, yet remarkably low—a phenomenon that they later termed “natural antibodies”. Later experiments also showed that thymectomized mice could no longer produce phage-specific antibodies, suggesting that antibody formation was a Th-dependent process (Dabrowska et al.)
One way to rationalize this is immune tolerance. If our body routinely suppresses antibodies against E. Coli and other bacteria, it follows that it wouldn’t mount a response against phages. Since bacterial and phage populations mirror each other, responding to one microbe and ignoring another makes no sense.
Or, perhaps we could cite the anti-inflammatory effect we described in our previous section.
Just as a single jammed conveyor belt can backlog an entire assembly line, it stands to reason that if phages can reliably suppress the initial steps of immunity (i.e. phagocytosis, cytokine release, immune cell recruitment), it stands to reason that this perturbation this could dampen the series of adaptive immune steps that follow.
It also seems that this effect seems to hold in the development of immunological memory.
In one experiment from Weisfuss et al., Escherichia and Pseudomonas phages were repeatedly administered to mouse models (at 0, 10, and 21 days after dosing) to probe their effects on a spectrum of immune markers (i.e. cell counts, IgG, IgM antibodies, etc). While repeated phage exposure induced measurable increases in IgG, the authors noted that this wasn’t nearly as significant as expected of a typical antiviral response3.

Interestingly, these findings held true when phages were inactivated by UV, but not when inactivated by agents that denatured the capsid. As expected, this suggests that the formation of antibodies hinges on the peripheral structure of the phage, as opposed to its genetic material.
From any angle, these findings are significant because they change our mental image of what phages are doing.
Our body on phages isn’t just more likely to ignore them in the moment — it’s more likely to forgive and forget past encounters.
Distilling our Evidence
In some way, forming opinions about research is like piecing together a satellite image.
With this analogy, a single paper resembles something like a pixel - the information it offers is important, but too tiny to be meaningful on its own. It’s only when we wade knees-deep into the literature and parse out themes that we begin to form convincing maps of a field.
To recap: we began this quest because we knew phages were safe (at least empirically) but didn’t know why. How come phages are antigenic, but not nearly as immunogenic as a typical virus?
As varied and contradictory as our literature was, it helped us nail down some key lessons.
Phages are widely distributed across the body and taken up by a variety of cells.
After engulfment, phages are readily fragmented even displayed on MHC receptors.
Despite this, cells don’t produce nearly the same profile of inflammatory cytokines as usual viral infection.
What follows is an attenuated antibody response and slower formation of immune memory against the phage.
Like any interesting evidence, these simple observations can produce a whirlwind of differing interpretations.
Still, from carefully turning over this evidence in my head, a few theories stand out.
Synthesizing Theories
What are the simplest explanations that let us make the most sense of these observations?
Desensitization
For starters, there’s the possibility of immune tolerance. This seems to align with the fact phages are consumed by cells, displayed on their surface, and produce antibodies — but these effects are inexplicably mild and don’t seem to affect therapeutic performance. In this case, this only spurs new questions.
Fundamentally, we’re still unsure how immune tolerance works at the molecular level. But even if we did, this still reveals gaping vulnerabilities in our knowledge.
If tolerance really is a key mechanism behind the safety of phages, does this mean our cells have specialized mechanisms to distinguish between phages and other viruses? And even if this were possible, how does recognition of phage lead to immune suppression?
Lack of cytotoxicity (AKA Harmlessness)
At this point, it’s worth noting another important distinction. Unlike most pathogenic human viruses, phages aren’t cytotoxic to human cells, and therefore don’t produce a surge in damage-associated molecule patterns (DAMPs) that would otherwise trigger severe immune responses4.
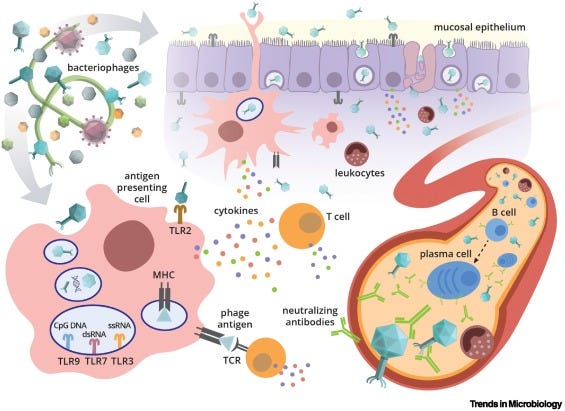
At first glance, this logic seems reasonable enough. After all, millions of non-human viruses readily pass through us without concern, presumably because they don’t directly damage our tissues. Through several (mostly unresolved) pathways, it appears that our immune system grants privileges to neutral or mutualistic microbes.
But I’d argue that this explanation isn’t completely satisfying. If you follow the logic further, the fact that phages don’t kill human cells only explains why our body is neutral to their presence.
It still doesn’t explain the key observation that cytokine profiles of phages are anti-inflammatory. While the fact that phages don’t damage our own tissues may be an important piece of our answer, I get the feeling that phages also engage in more dedicated, active processes to inhibit our conventional immune response.
Evolutionary Safety Package
Another passable theory is that phages potentially gained the ability to suppress inflammatory responses in order to protect their host bacteria in humans.
Perhaps this is best illustrated in the fact that phage exposure prevents phagocytosis of bacteria.
And this certainly wouldn’t be the only instance of phages protecting their host. For instance, our lab has large body of evidence suggesting that filamentous Pf4 phages in the lung protect Pseudomonas from antibiotics, presumably by forming protective films around them (Sweere et al.)
To this end, the fact that phages suppress immune response may not be intentional, but rather an evolutionary side-effect that allowed phages to ensure the survival of their host.
Of course, relationships between phages and their hosts take many forms, with interactions ranging from mutualistic, to adversarial, and virtually everything in between.
With this spectrum of phage-host interactions, I wonder if there’s a connection between the mutualism of a phage (i.e. the extent to which it can replicate without lysing its host) and its capacity to suppress the innate immune response.
One tangible test (which might even be possible computationally) is to plot the lytic-lysogenic activity of phages against their capacity to initiate inflammation in vivo5.
Ultimately, lysogeny is just one glancing indicator of a phage’s cooperation with its host. But my hunch is that we might see a positive connection between how temperate a phage is and how fiercely it defends its cognate bacteria from immune recognition.
If this relationship truly exists, then this might be another overlooked yet substantial route that phages evade immune recognition.
But to be painfully honest, I’m just not sure how to process (or trust) so much conflicting information without testing them empirically, without replicating these results for myself.
The ideas I’ve explored certainly aren’t comprehensive, and there’s always a chance that immune interactions are yet another example of how phages in nature transcend common sense.
Phaging the Future
Phages aren’t the antibiotics we always dreamt of. At least, not yet.
For phages to reach the clinic, they need to be standardized. The art of phage discovery, formulation, and testing needs to be turned into a science.
A cornerstone of this is understanding (not just theorizing, but properly, mechanistically knowing) how phages interact with the human immune system.
From speaking with professors, researchers, and industry leaders, one of the recurring pitfalls of phage therapy is its variability—both between batches and between people.
This isn’t just a question of phage selection or formulation, but human response. The same phage that works for one patient, for instance, could easily flop in another patient because they already have circulating antibodies against it.
Since we still don’t have a working biological understanding of these effects, the only way to determine the success of phage therapy is human exposure.
This already seems broken. In the next few decades, I predict that we will:
Develop models that can reliably predict patient-specific immune response based on phage identity, charge, concentration, charge, etc.
Use this information to personalize the formulation of phage therapies, ensuring best bioavailability and safety profile in patients
Engineer and apply the unique anti-inflammatory properties of phage beyond their current use as antimicrobials (i.e. immunosuppressants, alternatives to steroids, etc.)
A question that genuinely excites me is: how do we work backward from this future?
As varied as these outcomes are, perhaps you noticed that they all hinge on a better understanding of how phages interact with us.
It’s also clear that we can’t get to this point with the knowledge we have today. To me, it seems that the abyss we’re staring into isn’t just one of willpower or funding, but of cold, hard science.
We don’t know how phages are processed in our bodies. We don’t understand how they interact with immune pathways At least for now, knowledge is a clear, rate-limiting step. Many of the questions that remain can only be answered at the lab or in the clinic.
For now, this means a lot more old-fashioned, heads-down science. Science may have changed, but the way discoveries are made hasn’t.
I’m not sure what we’ll find, but I think you should look forward to it.
Footnotes
As early as the 1940s, researchers like Dubos et al. famously showed that Shigella phages administered intraperitoneally in mice could translocate into the brain within hours. Compared to other open questions, the localization and biodistribution of phages has been relatively well-studied.
Historically, the effect of phages on immune responses has been hard to probe. Even with modern purification techniques, phage lysates are often contaminated with bacterial endotoxins that can result in a confounding inflammatory response. Only in the past decade, with the advancement of techniques like HPLC, have studies like these begun exploring phage-immune interactions with low endotoxin or endotoxin-free lysates.
For some reason, I couldn’t seem to find data on how phages affected the number and specificity of memory T and B cells in vivo. I wonder if I’m missing something or if this is a genuine knowledge gap…
DAMPs are a broad class of substances that our immune system recognizes as proxies for tissue damage. This includes molecules like ATP and F-actin, which normally belong within cells but are crushed out en masse during acute damage (i.e. from a pathogen)
The clear issue with this experiment is that lytic phages are more likely to lyse bacteria than their lysogenic counterparts. This in itself could confound our results, since endotoxins flushed out from the bacteria could initiate an immune response on their own. One potential fix is to use phages cleared of their DNA (otherwise known as ghost particles) so we can evenly compare their immune response independent of their virulence.



